Entero
Enterococcus Evolution in Space: Environmental Adaptations, Antibiotic Resistance, and Clinical Implications (Entero)
Faculty Advisor/PI: Dr. Christopher E. Carr
Start Date: November, 2020
Current Status: First two GEARS flights took place in 2024; up to two more flights are expected.
Launch Provider: NASA / SpaceX CRS
Collaborators: Co-Is: Sarah Wallace and Aaron Burton, NASA Johnson Space Center (NASA); Michael Gilmore, Massachusetts Eye and Ear Infirmary (MEEI). Collaborators: Noelle Bryan, University of Colorado (Collaborator); Maria T. Zuber, Massachusetts Institute of Technology (MIT); Gary Ruvkun, Massachusetts General Hospital (MGH); Ralf Moeller, German Aerospace Center, Cologne, Germany (DLR); Elisabeth Grohmann, Beuth University of Applied Sciences, Berlin, Germany (Beuth) and an extended NASA team providing administrative and spaceflight support.
Enterococci are gram-positive bacteria that originated when our ancient animal ancestors emerged from the oceans to live on land, and brought their gut flora with them. Enterococcus faecalis (EF) and Enterococcus faecium, are common human commensals and harbor multidrug resistance. Both have been previously isolated on the International Space Station (ISS). Likely as a consequence of their evolutionary origins, enterococci show remarkable stress resistance within, but also outside, their human hosts. Their antibiotic resistance, coupled with tolerance to desiccation, starvation, and disinfection, make them potent pathogens in the built environment, and a risk to crew health during space missions.
Specific Aims: Here we propose a three-year PI team research program, including flight components, to: 1) Characterize the frequency and genomic identity of antibiotic resistant organisms, including enterococci, on the ISS; 2) Assess the evolutionary selective pressure of the space environment (microgravity, space radiation) using EF as a model system; and 3) Characterize the “natural” evolutionary history of EF on Earth and in space to reveal mechanisms of microbial adaptation including natural selection, and refine crew health risk assessment and countermeasures for future space missions. Each aim is addressed by a PI-led investigation.
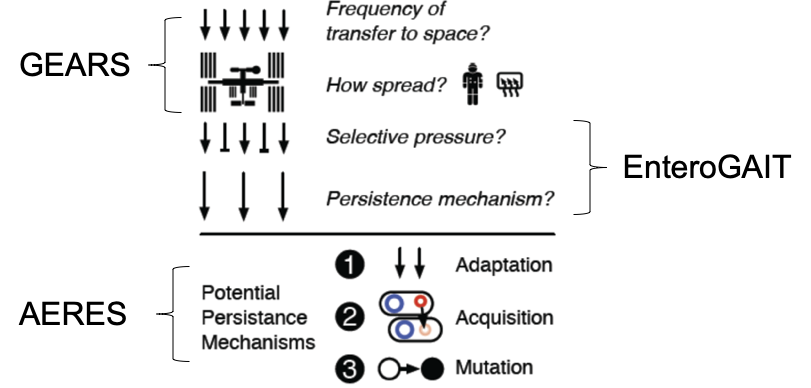
Genomic Enumeration of Antibiotic Resistance in Space (GEARS): Led by Co-PIs Burton and Wallace (NASA), this flight experiment will leverage existing ISS facilities to enable detection, species-level identification (via nanopore sequencing), and isolate selection (for Earth return and characterization), complementing ongoing routine microbial monitoring by focusing on the clinically-relevant subset of antibiotic resistant microbes. Two flights were conducted in 2024 resulting in the first metagenomic sequencing done in space and the first epigenomic sequencing conducted in space.
Enterococcus Growth Advantage on ISS via Tn-seq (EnteroGAIT): Led by Co-PI Gilmore (MEEI), this flight experiment will utilize the Bioculture System, currently undergoing validation on the ISS, to measure the selective pressure of the space environment on a defined microbial population: a library of EF mutants, created by transposon insertional mutagenesis, will undergo several rounds of growth and starvation on ISS with a synchronous ground control; selection is measured for each gene knockout by sequencing (Tn-Seq) and occurs on timescales far shorter than natural or experimental evolution. This experiment was de-prioritized for flight in late 2024.
Adaptation and Evolution of Resilient Enterococcus in Space (AERES): Led by PI Carr (MIT), this ground-based data and experiment-driven study will use an extensive collection of existing (>500) and newly generated (~100) whole genomes of Earth- and space-derived enterococci isolates to characterize horizontal gene transfer, mutation, and implications for community-acquired infection in space and on Earth. Genetic techniques will be used to establish causality.
Significance & Relevance: The proposed work will improve our fundamental understanding of microbial adaptation to the space environment and crew health risks. Our work directly addresses the solicitation research foci A.3.1.a (primary) and A.3.1.b (secondary) and the 2011 Decadal Survey recommendation to use the ISS as a Microbial Observatory (P1). Furthermore, our work addresses multiple Human Research Roadmap (HRR) gaps (MICRO-1 to MICRO-5 and Med01).
Funding: NASA Space Biology Award # 80NSSC21K0234 to C.E.C.